


Surgical Gloves
Sterile Latex Surgical Gloves Powdered (Sterile Pre-powdered)
Product Details
Surgery
General use
Pharmaceutical Manufacturing
Medical Procedures
Dental Procedures
Veterinary
- Lightly powdered with modified absorbable corn starch
- Fully anatomical design to reduce finger fatigue
- Textured surface which provides excellent wet and dry grip
- Premium softness and elasticity give a comfortable fit with excellent dexterity and flexibility.
- Palm-textured surface ensures better grip and control.
- High elasticity and strength for excellent comfort and protection.
- 100% inspected using automated pinhole test
- Passes viral penetration
- Passes Bacterial Endotoxin test
- Factory Standard of AQL 0.65 for pinholes
- Quality Standards manufactured under QSR
(GMP), 1SO 9001: 2015 & EN ISO 13485: 2016 Quality Management System conforms to Rule 06 as per MDCG 2021-24 & Annex VIII of MDR 2017/745 Class IIa and PPE Regulations 2016/425 Category III - ISO 14001: 2015 Environmental Managment System
- Also conforms to ASTM D3577, EN 455 – Part 1, 2, 3 & 4 and ISO 10282 Standards. Quality Control
| Type | Powder free Polymer Coated Surgical gloves |
| Design |
|
| Powder Content |
<2 mg/glove |
| Primary Material |
natural rubber latex (Type -1) |
| Sterilization | Ethylene Oxide (EO)/GAMMA |
| Colour | Creamy white to pale yellow in colour (Natural /colour) |
| Shelf Life | Products have shelf-life of 5 years from the date of manufacturing |
Sterile Latex Surgical Gloves Powdered (Sterile Pre-powdered)
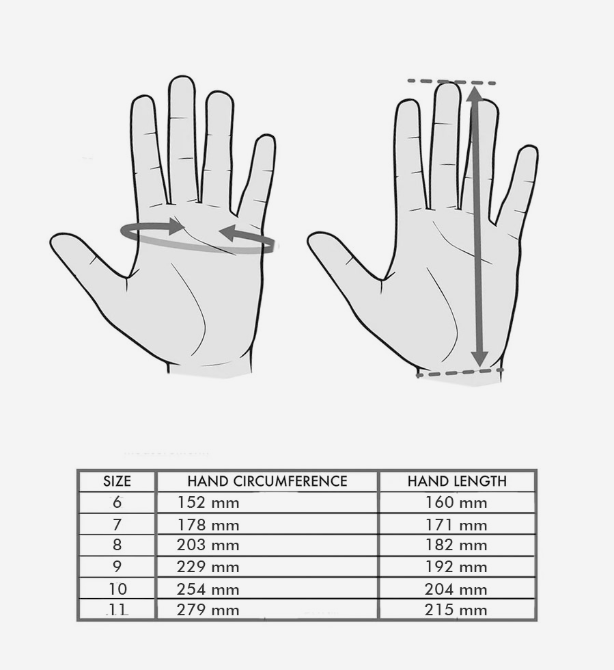
Size Chart
Warning: Undefined variable $featuredImage in /home2/stmarys1/public_html/wp-content/themes/wactheme/wac-template-parts/content-sizechart-popup.php on line 16

Surgical Gloves
Sterile Latex Surgical Gloves Powdered (Sterile Pre-powdered)
| Inches | CM |
|---|---|
| 5.5 | 14 |
| 6 | 15 |
| 6.5 | 17 |
| 7 | 18 |
| 7.5 | 19 |
| 8 | 20 |
| 8.5 | 22 |
| 9 | 23 |
| 9.5 | 24 |
How to measure yourself

Purpose: To ensure free from any chemical substances and safe to use any chemical substances and safe to.







